Pre-treatment of stainless steel plating
|
Stainless steel is composed of iron, nickel, chromium, titanium and other components. It is easy to form a thin, transparent and firmly attached passivation film on the surface. The surface is formed quickly, so according to the general steel parts plating process A coating with good adhesion cannot be obtained. Before electroplating of stainless steel, in addition to the oil removal and etching of general steel, activation pretreatment is usually required. Activation treatment is an important step to ensure sufficient adhesion of the electroplated layer. General activation treatment methods include cathodic activation method, immersion activation method and Zinc plating activation method, etc. (1) Cathodic activation treatment The cathodic activation treatment is due to the strong reduction of hydrogen on the surface of the cathode to prevent the formation of oxide film, thereby maintaining a fresh stainless steel surface. The process specifications are listed in Table 3-16. Table 3-16 Stainless steel cathode activation process specification
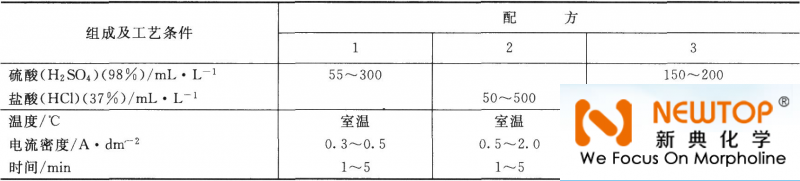 (2) Immersion activation treatment The process specification of stainless steel immersion activation treatment is listed in Table 3-17. Table 3-17 Stainless steel impregnation activation process specification
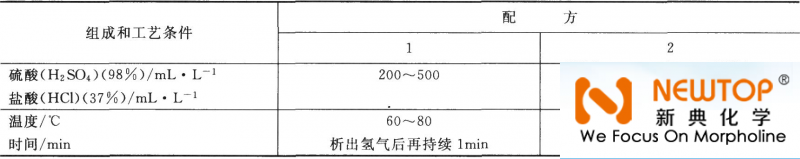 (3) Galvanizing activation treatment Plating a thin layer of metallic zinc on stainless steel, and then immersing it in reducing acid. Because the electrode potentials of zinc and stainless steel are different, a micro battery is formed in the medium to corrode and dissolve the zinc layer, and the stainless steel substrate acts as a cathode to precipitate The hydrogen on its surface reduces and activates the oxide film, thereby improving the bonding force of the covering layer. The specific operation process of zinc plating activation treatment is: ①Degrease the stainless steel substrate; ②Etch in 500mL/L hydrochloric acid for 5-10min. When the scale is thick, add hydrofluoric acid, sulfuric acid or phosphoric acid in the hydrochloric acid, and extend the etching time appropriately; ③Plating in an ordinary galvanizing bath for 1~2min, no more than 5min, then dezincing in 500mL/L hydrochloric acid or sulfuric acid, and then repeating the galvanizing and de-zincing, then other metals can be electroplated. |


