1-Naphthylamine-8-sulfonic acid
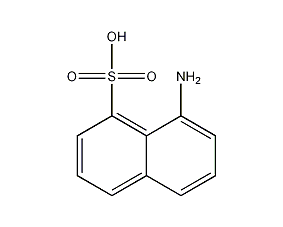
Structural formula
| Business number | 01T4 |
|---|---|
| Molecular formula | C10H9NO3S |
| Molecular weight | 223.25 |
| label |
Peripheral acid, 1,8-Clif acid, 8-amino-1-naphthalenesulfonic acid, forced acid, 1,8-Klev acid, 1-Naphthylamine-8-sulfonic acid, 8-Naphthylamine-1-sulfonic acid, 1,8-naphthylamine sulfonic acid, 8-aminonaphthalene-1-sulfonic acid, Peri acid, 8-Amino-1-naphthalenesulfonic acid, 8-Aminonaphthalene-1-sulphonic acid |
Numbering system
CAS number:82-75-7
MDL number:MFCD00035730
EINECS number:201-437-1
RTECS number:None
BRN number:983230
PubChem ID:None
Physical property data
1. Character: white needle-like crystal
2. Density (g/mL, 25/4℃): 1.72
3. Relative vapor density (g/mL, Air=1): Uncertain
4. Melting point (ºC): >350
5. Boiling point (ºC, normal pressure): Uncertain
6 . Boiling point (ºC, 5.2kPa): Uncertain
7. Refractive index: Uncertain
8. Flash point (ºC): Uncertain
9 . Specific rotation (º): Uncertain
10. Autoignition point or ignition temperature (ºC): Uncertain
11. Vapor pressure (kPa, 25ºC): Uncertain
12. Saturated vapor pressure (kPa, 60ºC): Uncertain
13. Heat of combustion (KJ/mol): Uncertain
14. Critical temperature (ºC): Uncertain
15. Critical pressure (KPa): Uncertain
16. Log value of oil-water (octanol/water) partition coefficient: Uncertain
17. Explosion upper limit (%, V/V): Uncertain
18. Explosion lower limit (%, V/V): Uncertain
19. Solubility : Soluble in glacial acetic acid, slightly soluble in hot water, very slightly soluble in cold water.
Toxicological data
None yet
Ecological data
None yet
Molecular structure data
1. Molar refractive index: 58.27
2. Molar volume (cm3/mol): 148.6
3. Isotonic specific volume (90.2K): 433.1
4. Surface tension (dyne/cm): 72.1
5. Polarizability (10-24cm3): 23.10
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): 1.2
2. Number of hydrogen bond donors: 2
3. Number of hydrogen bond acceptors: 4
4. Number of rotatable chemical bonds: 1
5. Topological molecular polar surface area (TPSA): 80.4
6. Number of heavy atoms: 15
7. Surface charge: 0
8. Complexity: 322
9. Number of isotope atoms: 0
10. Determine the number of atomic stereocenters : 0
11. Uncertain number of atomic stereocenters: 0
12. Determined number of chemical bond stereocenters: 0
13. Uncertain chemical bond formation Number of structural centers: 0
14, Number of covalent bond units: 1
Properties and stability
Contains one molecule of crystal water, which is lost when heated to 130°C.
Poisonous. Can cause poisoning if swallowed or absorbed through skin. Protective measures should be taken during the production process, good ventilation should be maintained at the production site, and operators should wear protective equipment.
Storage method
Should be sealed, dry and protected from light.
Packaged in 50kg plywood barrels or wood fiber round barrels lined with PVC bags. The mouth of the bag should be tied tightly and the lid of the bucket must be tightly sealed.
Synthesis method
The naphthalene method is mainly used. It is obtained by using naphthalene as raw material, low-temperature sulfonation with sulfuric acid, nitration with nitric acid, neutralization with magnesium carbonate, reduction of iron powder, and then acid precipitation and filtration. During the production of Klev’s acid, a mixed magnesium salt of aminonaphthalene sulfonate is obtained, which also contains magnesium salt of 1,8-aminonaphthalene sulfonate. These three isomer magnesium salts have different solubilities at different temperatures. During operation, the mixed solution (reducing solution) is first concentrated to a certain concentration, cooled to 30-32°C, and 1,6-aminonaphthalene sulfonic acid is precipitated first. Magnesium salt (after filtration, acid precipitation yields 1,6-Klef acid); the filtrate is partially acidified by adding sulfuric acid, stirred at 40-45°C for 12 hours, and 1-naphthylamine-8-sulfonic acid is obtained by filtration. The filtrate is further subjected to acid precipitation to obtain 1,7-Klev acid.
Purpose
Dye chemistry, synthesis of azo dyes. Organic Synthesis. Used to manufacture a series of aniline dyes and intermediates, such as phenyl peric acid, tolyl peric acid, amino C acid, Chicago acid, navy gray and sulfide dark green 3GW, etc.
extended-reading:https://www.cyclohexylamine.net/delayed-equilibrium-catalyst-dabco-catalyst/extended-reading:https://www.bdmaee.net/wp-content/uploads/2023/02/1-2-1.jpgextended-reading:https://www.bdmaee.net/dabco-dc1-delayed-catalyst-dabco-dc1-delayed-strong-gel-catalyst-dabco-dc1/extended-reading:https://www.newtopchem.com/archives/45120extended-reading:https://www.bdmaee.net/nn-dimethyl-ethanolamine-3/extended-reading:https://www.bdmaee.net/wp-content/uploads/2022/08/115-11.jpgextended-reading:https://www.newtopchem.com/archives/44203extended-reading:https://www.cyclohexylamine.net/catalyst-2033-tertiary-polyurethane-catalyst/extended-reading:https://www.bdmaee.net/wp-content/uploads/2022/08/134-1.jpgextended-reading:https://www.bdmaee.net/jeffcat-zr-50-catalyst-cas67151-63-7-huntsman/


